Surgeon-scientist Juliet Emamaullee, MD, PhD, spends much of her time seeing patients and performing pediatric liver transplants at Children’s Hospital Los Angeles. But you’ll also find her in the lab—studying how to make those new livers last longer in children.
“About half of the kids we transplant are transplanted before the age of 2,” explains Dr. Emamaullee. “I want their livers to last a lifetime.”
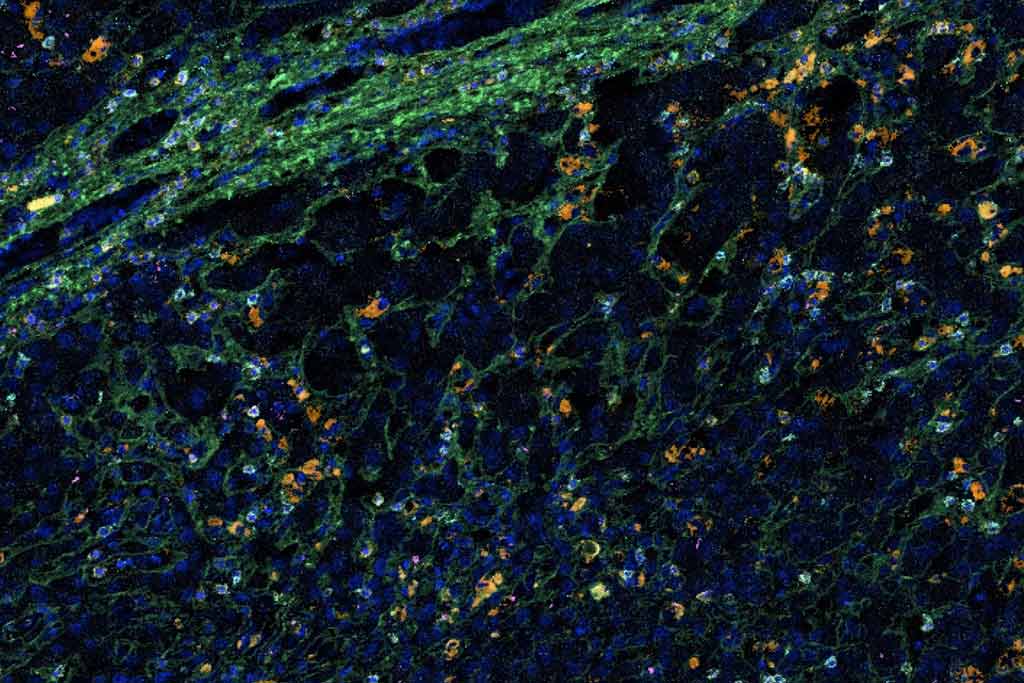
To achieve that, her lab is using a new technology called imaging mass cytometry to take a deeper look at the mechanisms of liver graft rejection—and develop novel ways to diagnose, treat and predict rejection in children. Her work is a partnership with the Single Cell, Sequencing, and CyTOF Core at The Saban Research Institute of Children’s Hospital Los Angeles. The Core is led by Shahab Asgharzadeh, MD, and Jeffrey Bender, MD.
Below, she talks about her research and what it could mean for the future of pediatric liver transplant.
What is imaging mass cytometry, and why is it critical to your research?
Imaging mass cytometry is a technology that allows us to examine up to 40 different proteins in a single cell—all at the same time—from just one tiny section of tissue. It’s a visual test, but then we use a whole bunch of computational biology to study each individual cell in a very discrete way. No one has been able to capture this level of data from tissue before.
We’re actually the first team to use mass cytometry in transplant research. We had to build an assay from the ground up. This is such a new technology, only about 50 papers have been published about it, and most of those are in the cancer literature. CHLA is one of the only facilities on the West Coast to even have this equipment.
What are you aiming to discover?
We want to understand what’s happening in the immune system inside the actual transplant, which hasn’t really been done before. We’ve already identified a few unique lymphocyte populations that might be important. We are now studying these cells in more detail to learn their origin and their role, as well as their potential as new treatment targets.
New treatments are critical because currently, we only have four or five medications to treat rejection, and those really haven’t changed in the past 30 years. But we’re also taking the same approach to find markers of rejection in both the tissue and the blood. The goal there is to develop a blood test that could diagnose rejection without a needle biopsy, as well as predict a patient’s rejection risk and response to treatment.
Why is predicting rejection risk important?
We know that a percentage of liver transplant recipients may not need immune-blocking medication at all, after a period of time. We just don’t know who those patients are.
If we could identify tissue and blood markers that predict rejection, then some of the low-risk patients possibly wouldn’t have to take these medications, which come with a lot of side effects. That’s especially important for children because they have to take these medications their entire lives, from a young age.
What are the challenges of working with a new technology like mass cytometry?
The analysis is very difficult. There isn’t a simple software program where you can just upload the data and work with it. We had to build our whole analysis pipeline to figure out how to handle the data. It’s very powerful, what you can do with it. But it’s fairly complicated.
You joined Children’s Hospital Los Angeles three years ago. What makes this a good place for your research?
First, we have one of the busiest pediatric liver transplant programs in North America. We do between 25 and 30 pediatric liver transplants a year, a third of which are living donor liver transplants. So, we have a large population of patients to study, and that population is also very ethnically diverse. And then we have access to imaging mass cytometry. It’s the perfect situation. We have all the pieces in place to get meaningful results.
What motivates you to do research?
I fell in love with science before I fell in love with medicine. I trained first in my PhD, then I did my MD and a postdoc. I think it’s so important to have clinician-scientists who are directly involved in patient care. We know what the problems are because we see them every day. And we have that relationship with our patients.
As a surgeon, after a while, all the successes kind of fade into the background. The patients you struggle to get a good result for are the ones who keep you up at night. For me, having the lab is another pathway of how to find an answer for those patients. Rather than just saying, “You have rejection, we tried everything, you’re going to need a new transplant,” I want to learn something. I want to make it better for the next patient.
Dr. Emamaullee’s research is supported by a $1.3 million grant from the National Cancer Institute, as well as grants from the American Society of Transplant Surgeons and the American Association for the Study of Liver Diseases (AASLD).