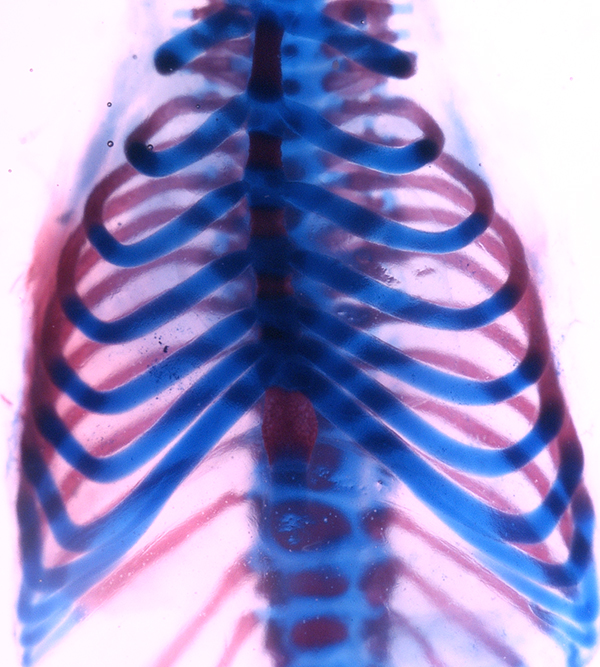
Scientists from the USC Stem Cell lab of Francesca Mariani recently shared a recipe for ribs, and it doesn’t even require barbecue sauce.
In a new publication in the journal eLife, first authors Jennifer Fogel from USC, Daniel Lakeland from Lakeland Applied Sciences and colleagues examine the development of the vertebrate ribcage, which supports the body, protects the internal organs and enables life on land.
In the study, the authors describe a simple computational tool that models the choices cells make while the ribcage develops in the early mouse embryo. Some cells choose to become the bony section of each rib that connects to the spine, while other cells choose to form the cartilage section of each rib that joins the sternum. Understanding this process required the team to integrate the effects of cell growth, cell death, and cell communication into their computational tool in order to gain insights into how the skeleton forms.
Using the model, the scientists propose that the different levels of a secreted protein called Hedgehog (Hh) are important for cells to make the decision to form bone or cartilage. High levels of Hh bias the cells towards making the bone component. As Hh travels further away from its source at the midline of the back, concentrations of Hh drop. Lower concentrations bias the cells towards making the more distant cartilage component of each rib.
Each cell’s decision to contribute to the bone or cartilage component is likely locked-in early when the embryo is very small, and maintained as the embryo grows exponentially.
“Our study suggests that regardless of whether an embryo gives rise to a large elephant or a small mouse, that the rib skeleton has already organized itself while the embryo is smaller than a grain of rice,” explained Mariani, assistant professor of stem cell biology and regenerative medicine and principal investigator in the Eli and Edythe Broad Center for Regenerative Medicine and Stem Cell Research at USC. “In addition, the modeling approach we developed can be used to understand the challenges of building new tissues in adults after injury.”
In Kyoung Mah from USC also contributed to the study. Funding came from the University of Southern California, and NIH NIAMS. Fogel was supported by a postdoctoral fellowship from the California Institute for Regenerative Medicine.
