A multidisciplinary team of researchers from the University of Southern California (USC) Roski Eye Institute, Children’s Hospital Los Angeles (CHLA) and the USC Viterbi School of Engineering used stem cell-derived retinal organoids and enhanced imaging technologies to assess an important retinal development and disease model in their cover story published this month in Investigative Ophthalmology & Visual Science.
The team, headed by David Cobrinik, associate professor of research ophthalmology at the USC Roski Eye Institute and USC Stem Cell principal investigator, successfully live imaged the developing retinal organoids, which are 3D biomimetic tissue models that possess similar architecture and cellular composition to that of the retina, or the light sensing tissue found in the back of the eye. The retina is a complex well-organized neural tissue, consisting of multiple layers of cells such as photoreceptors that are critical to healthy vision.
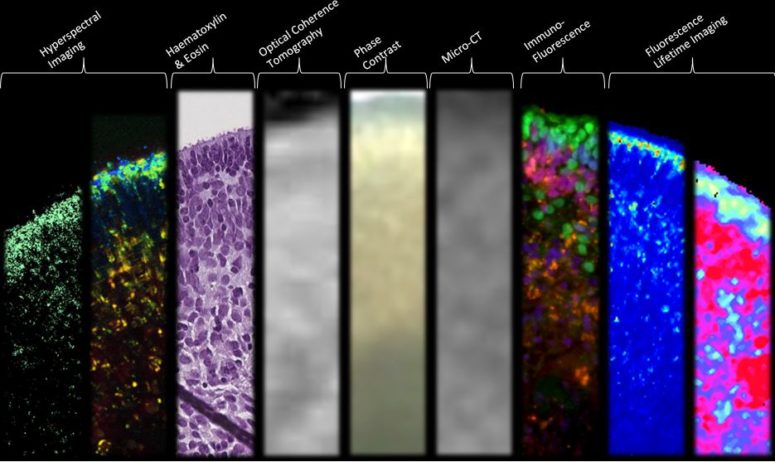
Funded by the National Institutes of Health (NIH), the research article, “Structural and Functional Characterization of Human Stem-Cell-Derived Retinal Organoids by Live Imaging,” describes how retinal organoids, are imaged at various stages of development through advanced imaging technologies including optical coherence tomography (OCT), hyperspectral imaging (HSpec), and fluorescence-lifetime imaging (FLIM).
“Understanding how the normal retina develops is crucial to discovering the root causes of a wide range of genetic retinal diseases,” says Cobrinik, who is also an investigator with The Saban Research Institute of CHLA. “Retinal organoids can provide greater understanding of retinal disease mechanisms and may help us discover new treatments for those devastated by sight-threatening conditions.”
Andrew Browne initiated and performed the investigation during his residency at the USC Roski Eye Institute. The methodology incorporated non-invasive multimodal imaging techniques in collaboration with Scott Fraser, provost professor of biomedical engineering, professor of stem cell biology and regenerative medicine, and director of USC’s Translational Imaging Center. The imaging analysis of the retinal organoid models detected metabolic activity as well as structural features of these models.
The researchers gained insight into the cellular metabolism and structural formation of retinal organoids by assessing developmental changes over a period of 46–151 days. For example, through the innovative FLIM and HSpec imaging modalities performed by Browne, Fraser and postdoctoral researcher Cosimo Arnesano, photoreceptor development was observed by quantitative imaging of retinol and retinoic acid metabolites. FLIM, HSpec and OCT imaging techniques confirmed that live retinal organoids exhibit specific lamellar or 3D architecture similar to that of the native retina.
“Unlike other methodologies, like histology or immunostaining that result in destruction of tissue, FLIM and HSpec are noninvasive imaging modalities, provide valuable structural and functional information, and are thus powerful tools in the real-time evaluation of the early stages of retinal development,” said Browne.
Similar to the native retina, the retinal organoids, developed by CHLA researchers Jennifer Aparicio and Thomas Lee, chief of CHLA pediatric ophthalmology and director of the CHLA Vision Center as well as USC Roski Eye Institute faculty, were created to better understand retinal genesis.
CHLA and the Keck School of Medicine of USC, of which USC Roski Eye Institute is a part, have an 85-year history in collaborating on research and patient care. This research team is among a small number of teams nationwide that create retinal organoids to model retinal development and disease. Cobrinik’s research focuses on understanding the development of retinoblastoma, the most common ocular tumor in children. Having identified the cell of origin for retinoblastoma, which was published in Nature, Cobrinik’s team determined that the loss of the retinoblastoma tumor suppressor protein (pRB) causes abnormal cone photoreceptor cell proliferation followed by cancer.
Note: This study was supported by grants from the National Institutes of Health (NIH), Bethesda, MD. All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported. PMID: 28672397, PMCID: PMC5495152, DOI: 10.1167/iovs.16-20796
