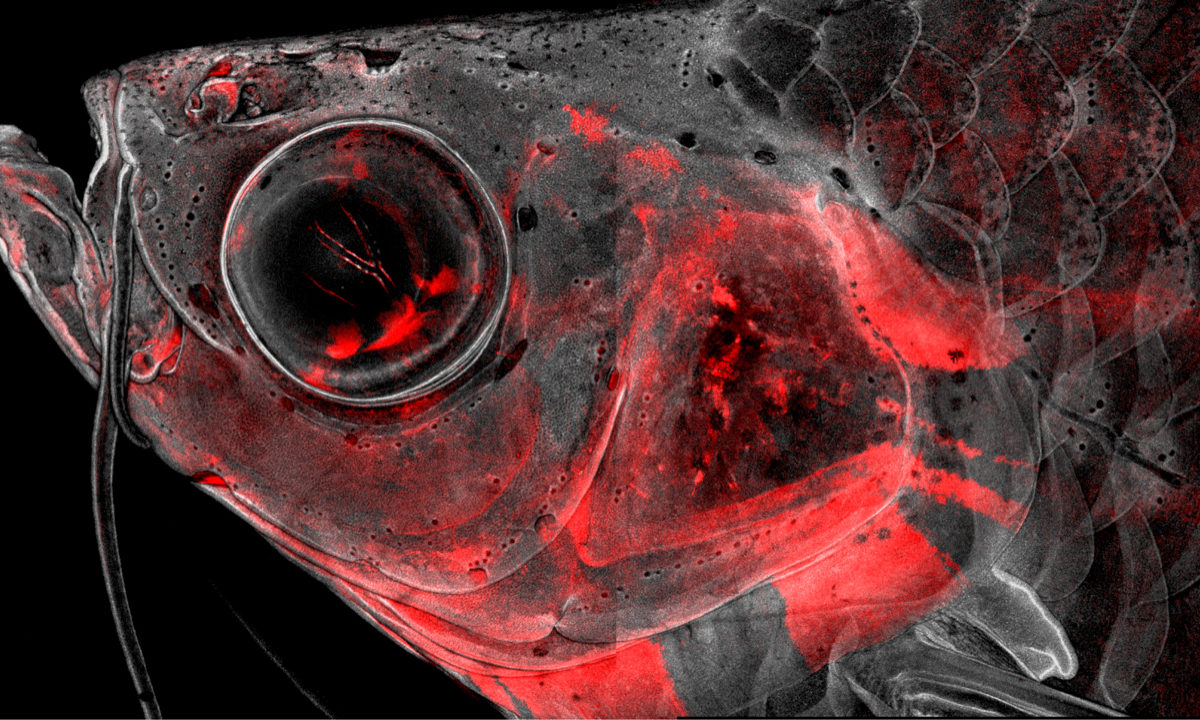
Cranial neural crest cells, or CNCCs, contribute to many more body parts than their humble name suggests. These remarkable stem cells not only form most of the skull and facial skeleton in all vertebrates ranging from fish to humans, but also can generate everything from gills to the cornea. To understand this versatility, scientists from the lab of Gage Crump created a series of atlases over time to understand the molecular decisions by which CNCCs commit to forming specific tissues in developing zebrafish. Their findings, published in Nature Communications, may provide new insights into normal head development, as well as craniofacial birth defects.
“CNCCs have long fascinated biologists by the incredible diversity of cell types they can generate. By studying this process in the genetically tractable zebrafish, we have identified many of the potential switches that allow CNCCs to form these very different cell types,” said Gage Crump, professor of stem cell biology and regenerative medicine at the Keck School of Medicine of USC.
Led by postdoc Peter Fabian and PhD students Kuo-Chang Tseng, Mathi Thiruppathy and Claire Arata, the team of scientists permanently labeled CNCCs with a red fluorescent protein to keep track of which cell types came from CNCCs throughout the lifetime of zebrafish. They then used a powerful type of approach, known as “single-cell genomics,” to identify the complete set of active genes and the organization of the DNA across hundreds of thousands of individual CNCCs. The massive quantity of data generated required the scientists to develop a new computational tool to make sense of it.
“We created a type of computational analysis that we called ‘Constellations,’ because the final visual output of the technique is reminiscent of constellations of stars in the sky,” said Fabian. “In contrast to astrology, our Constellations algorithm really can predict the future of cells and reveal the key genes that likely control their development.”
Through this new bioinformatic approach, the team discovered that CNCCs do not start out with all the information required to make the huge diversity of cell types. Instead, only after they disperse throughout the embryo do CNCCs begin reorganizing their genetic material in preparation for becoming specific tissues. Constellations accurately identified genetic signs that point to these specific destinies for CNCCs. Real-life experiments confirmed that Constellations correctly pinpointed the role of a family of “FOX” genes in facial cartilage formation, and a previously unappreciated function for “GATA” genes in the formation of gill respiratory cell types that allow fish to breathe.
“By conducting one of the most comprehensive single-cell studies of a vertebrate cell population to date, we not only gained significant insights into the development of the vertebrate head, but also created a broadly useful computational tool for studying the development and regeneration of organ systems throughout the body,” said Crump.
Additional co-authors in the Crump Lab included PhD student Hung-Jhen Chen, postdoc Joanna Smeeton, and research technician Nellie Nelson. Smeeton is now an assistant professor at Columbia University, and Nelson is a PhD student at the University of California, Irvine.
The team has made their findings publicly available through the FaceBase Consortium, a USC-led data-sharing community for craniofacial and dental researchers, supported by the National Institute of Dental and Craniofacial Research (NIDCR).
The research was federally funded by the National Institutes of Health (grants NIDCR R35 DE027550, NIDCR K99 DE029858, NIDCR F31 DE029682-02, NICHD T32 HD060549).
