A person’s face is the first thing that others see, and much remains unknown about how it forms — or malforms — during early development. Recently, Chong Pyo Choe, a senior postdoctoral fellow working in the lab of USC stem cell researcher Gage Crump, has begun to unwind these mysteries.
In a September study published in the journal Development, Choe and Crump describe how a mutation in a gene called TBX1 causes the facial and other deformities associated with DiGeorge syndrome.
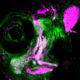
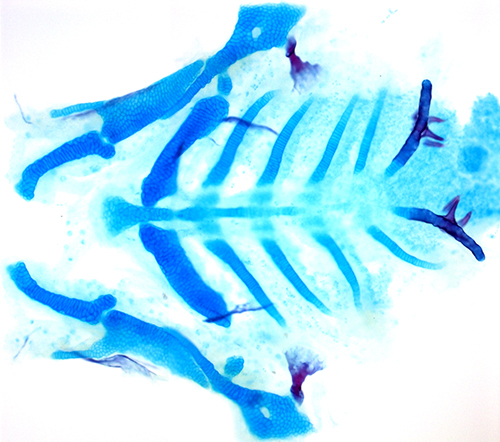
During prenatal development, a series of segments form that eventually organize many features of the face. These segments, or “pouches,” are composed of a type of specialized tissue called epithelium, which also forms the skin, glands and linings of organs such as the lungs, heart and intestines.
In mice and zebrafish with TBX1 mutations, these pouches never properly develop and the face is deformed, mimicking the severe facial defects typical of DiGeorge syndrome.
By using sophisticated time-lapse imaging, Crump and Choe observed how this happens in both normal and abnormal development. TBX1 works by activating additional genes, including one called Fgf8a that attracts pouch-forming cells to move to the correct locations. This enables the growing pouches to take shape.
“Whereas it has been recognized that mutations in TBX1 underlie DiGeorge syndrome in patients, our study reveals how this master control gene works to organize the complex cellular rearrangements that build the face,” said Crump, associate professor and principal investigator at the Eli and Edythe Broad Center for Regenerative Medicine and Stem Cell Research at USC.
Funding for this study came from a National Institute of Dental and Craniofacial Research (NIDCR) grant (R01DE022572) and a California Institute for Regenerative Medicine (CIRM) training fellowship.