If you lose a limb, it’s lost for life. If you damage a kidney, you won’t grow a new one. And if you have a heart attack, the scars are there to stay.
But regenerative medicine is poised to change all of this. Building new tissue is within sight, and USC scientists are among the field’s pioneers.
More than 100 scientists, engineers and doctors are united under what’s called the USC Stem Cell initiative. They’re already moving stem cells out of the lab and toward patient care. The potential is exciting: USC researchers have contributed to clinical trials of stem cell approaches to treating colorectal cancer, spinal cord injury, vision problems, HIV/AIDS and Alzheimer’s disease. They’ve also used stem cells to uncover important insights about kidney disease, ALS, arthritis, Zika virus, birth defects and a wide variety of injuries.
Major funders and USC donors have provided hundreds of millions of dollars to support the work. That investment and vote of confidence enables USC Stem Cell scientists to collaborate with other leading universities, biotech companies and key partners to translate their laboratory discoveries into patient cures.
It hasn’t been easy. Scientists are evaluating some stem cell-based therapies through clinical trials, but so far, few treatments have made it to patients. Beyond scientific inspiration, taking treatments from lab bench to patient bedside requires immense amounts of time, money and, sometimes, a bit of luck. It also means working together with other scientists across boundaries.
“Regenerative medicine is still a relatively young field, and it’s still early days,” says Andy McMahon, director of the Eli and Edythe Broad Center for Regenerative Medicine and Stem Cell Research at USC. “When it comes to that final phase of translating stem cell discoveries into clinical therapies for patients, it won’t be individual universities working in isolation. It will be multi-institutional collaborations with our neighbors that will transform medicine over the course of the 21st century.”
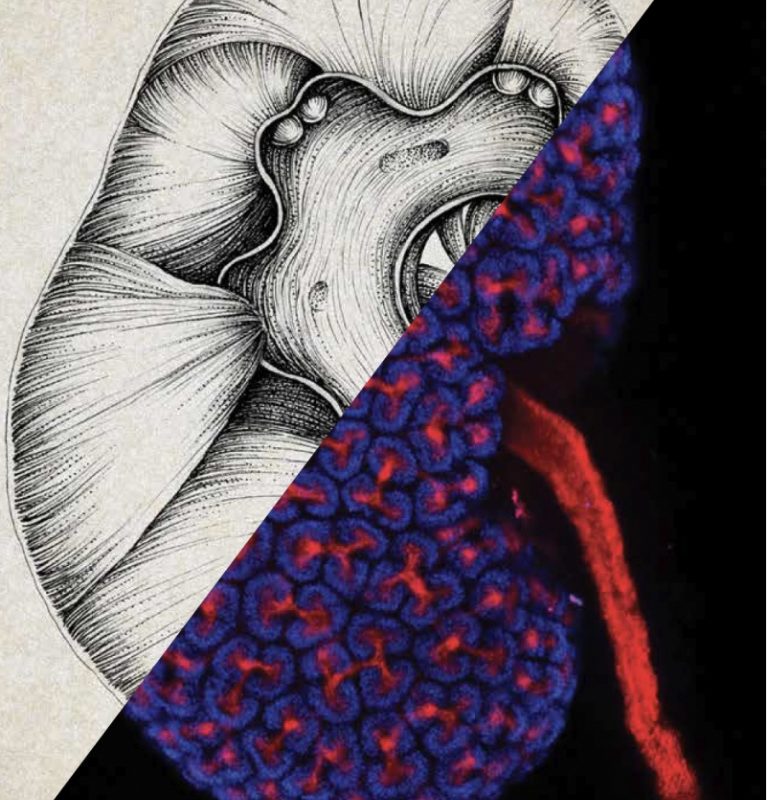
The Kidney in Miniature
So far, scientists haven’t been able to create complete adult human kidneys—they’re too complex.
At USC, though, McMahon’s lab is coaxing stem cells to organize themselves into simplified, mini versions of this elaborate organ.
Each healthy human kidney is made up of a million cellular filters called nephrons, which pull wastes out of blood, among other responsibilities. McMahon and his colleagues are making tiny organs (scientists dub them “organoids”) composed of a single nephron—a convenient size for testing potential drugs.
With help from USC’s Chang Stem Cell Engineering Facility, McMahon’s lab has successfully produced organoids carrying the same genetic mutation that causes polycystic kidney disease, the most common genetic cause for kidney failure. Because kidney organoids develop cysts similar to those seen in the disease, scientists can observe how the disease progresses and develop therapies that may halt or reverse symptoms.
Zhongwei Li, an assistant professor of medicine, and stem cell biology and regenerative medicine, is also hard at work growing kidney organoids. There are only 18,000 donor kidneys available each year for more than 400,000 patients who need them, Li explains. He ultimately wants to create organs for transplantation using special stem cellsprogenitor cells that could develop and organize themselves into kidney tissue.
“USC is a perfect place to study the kidney,” says Li, an assistant professor of medicine, and stem cell biology and regenerative medicine.
Healing Hearts
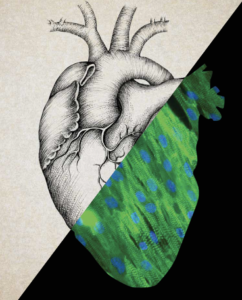
If you worry about dying in an earthquake, shark attack or lightning strike, don’t waste your energy. You’re far more likely to die of heart disease. Every year, about 610,000 people in the U.S. die of heart disease. That’s one in four deaths. And heart disease is the leading cause of death worldwide.
Cardiac tissue that has died after a heart attack doesn’t come back—it just forms a scar. Studies have shown that doctors can safely inject stem cells into damaged heart tissue, but there’s no clear sign that these injections restore the heart.
At USC, two stem cell researchers are tackling heart repair from other directions.
In the lab of Henry Sucov, researchers aim to harness the heart’s innate ability to heal. They’re studying a regenerative type of heart muscle cell called a mononuclear diploid cardiomyocyte. Newborns have large numbers of these cells, but adults have relatively few, so the adult body has trouble regenerating heart tissue after injury.
When they looked for these cells in mice, they found that some mice had more of these cells than other mice did. They traced that variation to a gene called Tnni3k. Their research suggests that blocking the gene might boost numbers of regenerative cells.
If scientists can create prescription drugs to modulate the activity of the gene, these medications could encourage more regenerative cells to develop in the heart, says Sucov, a professor of stem cell biology and regenerative medicine, integrative anatomical sciences, and biochemistry and molecular biology. “This could improve the potential for regeneration in adult hearts, as a preventive strategy for those who may be at risk for heart failure.”
In Megan McCain’s lab at the USC Viterbi School of Engineering, researchers are building human heart tissue. They not only study how the heart tissue works, but also use it to test how it responds to potential drugs.
The work poses problems that call for the mindset of an engineer. It turns out that heart muscle cells don’t fully mature in the typical laboratory environment for growing cells—a petri dish filled with warm, nutritious liquid. To develop properly, heart muscle cells need to get some exercise by contracting in the rhythm of a beating heart. To do this, they need structure and resistance, which the lab’s researchers provide in the form of a tiny scaffold called a chip.
This “heart on a chip” reproduces natural human heart tissue on a small scale in the lab.
Ultimately, McCain hopes the technology contributes to precision medicine. Scientists could test medications on a patient’s own heart tissue on a chip. Eventually, this could enable doctors to customize dosing and choose drugs that pose the fewest side effects to each patient.
Stronger Bones
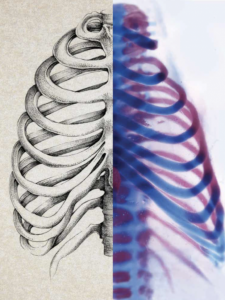
According to common wisdom, bones heal. In reality, every year about 5 million people in the U.S. sustain fractures that fail to mend. From elderly people undergoing total hip or knee replacements to soldiers injured by explosions or gunshots, many patients have bone defects that are too severe to repair. To complicate matters, everything from diabetes to the normal aging process can undermine bone’s ability to heal.
USC researchers hope to one day use stem cells to build new bone in patients with severe or non-healing injuries. Jay R. Lieberman, who chairs the Department of Orthopaedic Surgery at the Keck School, teamed up with Gage Crump and Francesca Mariani, two faculty members from the Department of Stem Cell Biology and Regenerative Medicine, to advance the science.
The team has made a promising start in the lab. They discovered that healing bone requires a special type of repair cell, which they named an ossifying chondrocyte. Now the researchers are studying a substance that stimulates these repair cells to fix bone.
Unlocking Genetic Diabetes
Nearly 10 percent of Americans, or 30 million people, have a form of diabetes. Diabetes happens when glucose levels rise in the blood. Insulin, a hormone made by the pancreas, helps the body pull glucose from blood and into the cells where it’s needed. But sometimes the pancreas doesn’t make enough insulin or the body can’t use insulin well.
Oftentimes, in diabetes, the special cells in the pancreas that make insulin—called beta cells—are attacked by the immune system or wear out. Researchers worldwide are looking at ways to rebuild them.
At Children’s Hospital Los Angeles (CHLA), researcher Senta Georgia aims to use stem cells to help patients with genetic forms of diabetes.
Her lab is focusing on a young CHLA patient with a rare genetic disease known as enteric anendocrinosis. The disease causes chronic diarrhea because patients lack certain gastrointestinal cells that produce hormones, and they eventually lose their beta cells as well, causing diabetes.
With the help of USC’s Chang Stem Cell Engineering Facility, Georgia’s team took stem cells derived from the patient’s skin and edited the cells’ genome to fix the genetic mutation behind the problem. They then used these genetically corrected stem cells to generate new insulin-producing cells.
The goal is to eventually transplant these insulin-producing cells back into the patient to reverse the diabetes—providing a tailor-made cell replacement therapy.
“We hope that this study can create a precedent for how to generate new insulin cells for patients with genetic forms of diabetes,” says Georgia, assistant professor of pediatrics and stem cell biology and regenerative medicine at the Keck School of Medicine of USC.
Fresh Faces
“Our faces are our identities, and the first thing you see when you look at someone is his or her face,” says Yang Chai, director of the Center for Craniofacial Molecular Biology at the Herman Ostrow School of Dentistry of USC. But when someone has a cleft lip or other facial deformity or trauma, it can be devastating.
Chai aims to find treatments for some of the most common craniofacial birth defects and injuries. To do that, he has tapped into a rich source of stem cells: the pulpy interior of the teeth.
Fueled by a $12 million grant from the National Institutes of Health (NIH), he’s working with researchers from the Keck School of Medicine and institutions from Stanford to City of Hope on the project.
They’ve already used these stem cells to generate the unique, high-density bone that makes up the skull. If these stem cells can effectively repair four-centimeter holes in the skulls of animals, the research project will advance the treatment into a clinical trial for patients with bone deficiencies due to injuries, dental problems or birth defects.
One birth defect USC scientists are tackling is called craniosynostosis. The rare-but-serious problem occurs when sections of a baby’s skull fuse together at joints called sutures, restricting the developing brain and disrupting vision, sleep, eating and IQ. To treat this condition, growing children must undergo repeated skull-expanding surgeries—which are as dangerous and painful as they sound.
Chai is one of at least a dozen USC stem cell researchers working to help these children. His lab has already identified a critical stem cell population that normally resides in the skull sutures, and discovered how to manipulate these stem cells to form new sutures in mice.
“This is something that truly has to be done through a collaborative effort,” Chai says. “USC provides the best environment for collaborative research, which has led to NIH funding and publications as the result of these collaborations. These collaborative studies will fundamentally change the way to provide health care to our patients.”
