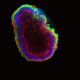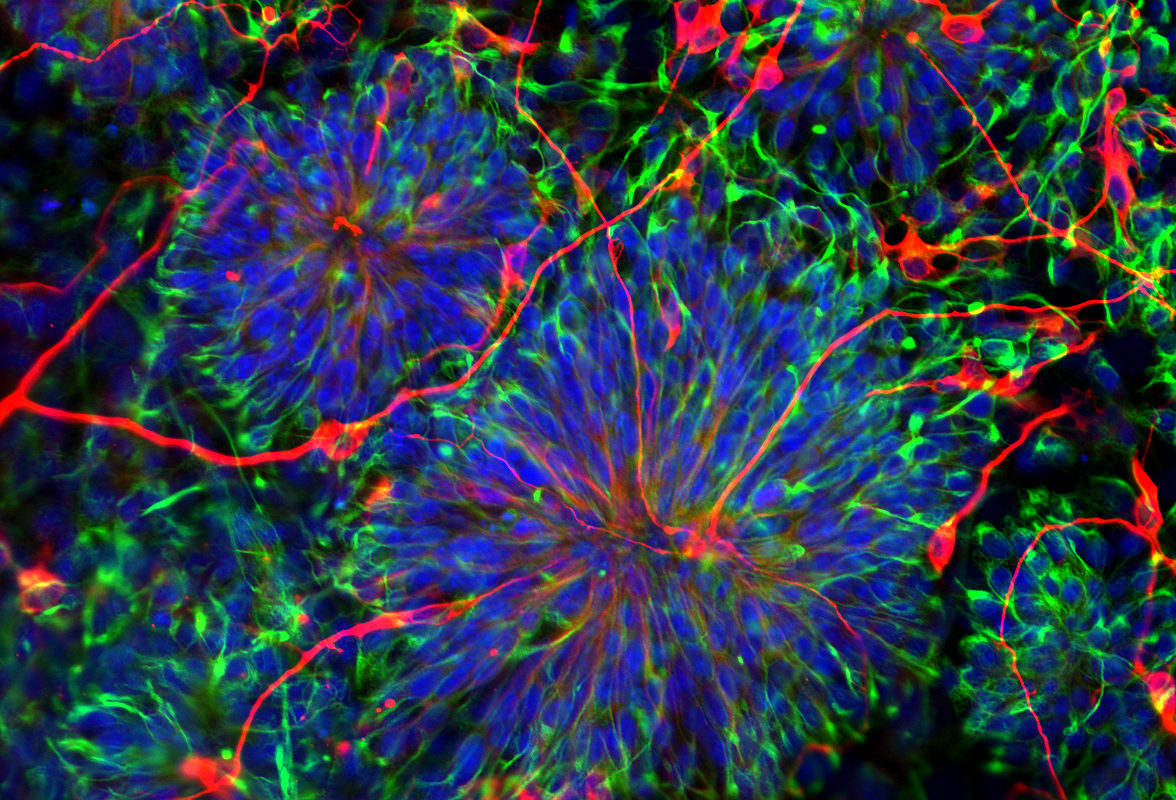
When it comes to stem cells, too much of a good thing isn’t wonderful: producing too many new stem cells may lead to cancer; producing too few inhibits the repair and maintenance of the body.
In a paper published in Stem Cell Reports, USC researcher In Kyoung Mah from the lab of Francesca Mariani and colleagues at the University of California, San Diego, (UCSD) describe a key gene in maintaining this critical balance between producing too many and too few stem cells. Called Prkci, the gene influences whether stem cells self-renew to produce more stem cells, or differentiate into more specialized cell types, such as blood or nerves.
In their experiments, the team grew mouse embryonic stem cells, which lacked Prkci, into embryo-like structures in the laboratory. Without Prkci, the stem cells favored self-renewal, generating large numbers of stem cells and, subsequently, an abundance of secondary structures.
Upon closer inspection, the stem cells lacking Prkci had many activated genes typical of stem cells, and some activated genes typical of neural, cardiac and blood-forming cells. Therefore, the loss of Prkci can also encourage stem cells to differentiate into the progenitor cells that form neurons, heart muscle and blood.
Prkci achieves these effects by activating or deactivating a well-known group of interacting genes that are part of the “Notch signaling pathway.” In the absence of Prkci, the Notch pathway produces a protein that signals to stem cells to make more stem cells. In the presence of Prkci, the Notch pathway remains silent, and stem cells differentiate into specific cell types.
These findings have implications for developing patient therapies. Even though Prkci can be active in certain skin cancers, inhibiting it might lead to unintended consequences, such as tumor overgrowth. However, for patients with certain injuries or diseases, it could be therapeutic to use small molecule inhibitors to block the activity of Prkci, thus boosting stem cell production.
“We expect that our findings will be applicable in diverse contexts and make it possible to easily generate stem cells that have typically been difficult to generate,” said Francesca Mariani, principal investigator at the Eli and Edythe Broad Center for Regenerative Medicine and Stem Cell Research at USC.
Additional co-authors on the study include Rachel Soloff and Stephen Hedrick from UCSD, and the research was supported by USC and the Robert E. and May R. Wright Foundation.