Design redundancy is not only an invention of engineers for building machines, but also a principle of nature for designing organisms. This principle is at play in the regulation of the genes responsible for directing stem cells to multiply themselves in the developing mouse embryo, as described in a new study in Science Advances.
In the study, scientists Oliver Bell, Jorge Zepeda-Martinez, and their collaborators from the Vienna BioCenter and USC studied the “silencing” of key genes that direct stem cells to differentiate into specific cell types or lineages. When these lineage-specific genes are silenced, the stem cells produce more stem cells, enabling the normal embryonic development of a mouse.
Silencing these genes involves a group of proteins called Polycomb repressive complexes, or PRCs. The PRCs make what are known as epigenetic changes, which reduce the activity of the lineage-specific genes that would commit a stem cell to becoming a more specialized cell type.
The redundancy is that there are two separate groups of PRCs, and both groups independently and simultaneously work to silence the same lineage-specific genes. If PRC group one stops working, then group two can handle the job. If PRC group two fails, then group one is a capable backup.
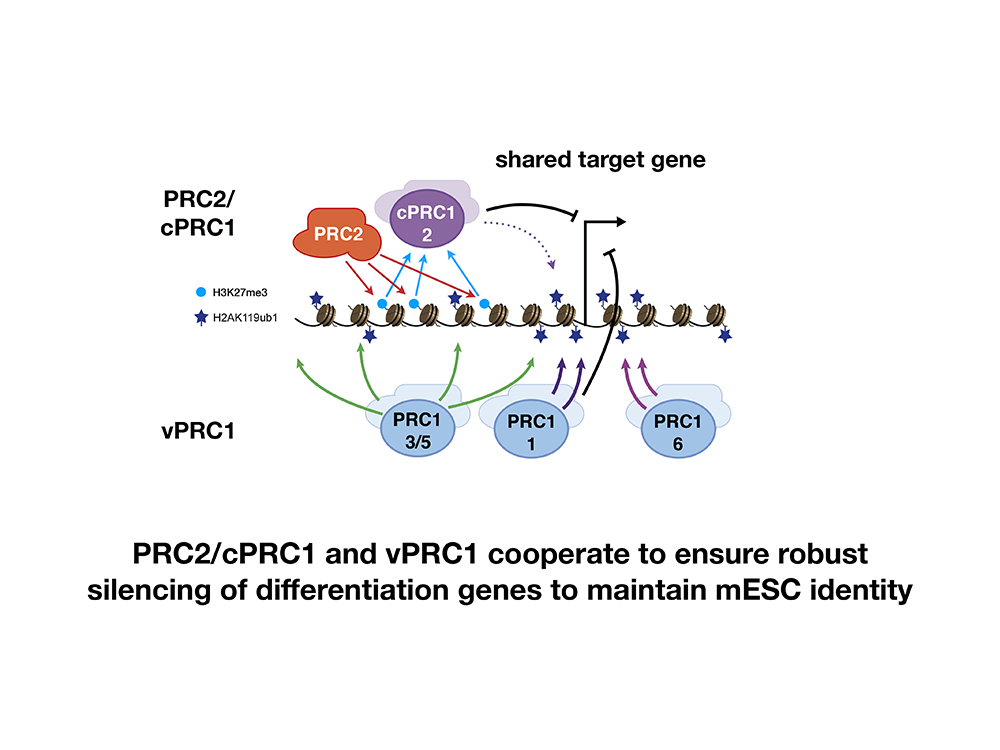
“Our results reconcile previous observations that one of the PRCs, namely PRC2, is not required for the self-renewal of mouse embryonic stem cells. We now show that PRC2 is critical for directing cPRC1 activity to maintain repression of lineage-specific target genes, when vPRC1 function is compromised,” said Bell. “Thus, the PRCs coordinate redundant mechanisms that ensure robust repression of key lineage-specification genes not only for differentiation, but also for maintaining the identity of mouse embryonic stem cells.”
Additional co-authors include: Carina Pribitzer, Jingkui Wang, Thomas R. Burkard, Brian Reichholf, Julian Jude, Hagar F. Moussa, and Johannes Zuber from the Vienna BioCenter; Daniel Bsteh from the Vienna BioCenter and USC; and Silvia Golumbeanu, Qing (Sunny) Zhao, and Suhn Kyong Rhie from USC.
Five percent of this research was federally funded by a U.S. National Institutes of Health grant (K01CA229995). Ninety-five percent was funded by other sources: the Austrian Academy of Sciences, the New Frontiers Group of the Austrian Academy of Sciences (NFG-05), the Human Frontiers Science Programme Career Development Award (CDA00036/2014-C), the USC Norris Comprehensive Cancer Center, and a Boehringer Ingelheim Fonds PhD Fellowship.